Abstract
Background:Knowledge of prognostic factors for recurrence in adult and pediatric VTE is limited. Elevated plasma levels of D-dimer (a marker of coagulation activation) following a 6-month course of anticoagulation in adults with unprovoked VTE are associated with a heightened risk of VTE recurrence, warranting prolonged therapy. In patients with provoked VTE, and in young VTE patients more generally, prognostic factors remain largely undefined. Recently, using a global assay of clot formation and lysis on plasma samples collected serially over time, we observed a phenomenon of rebound hypercoagulability at 3 months post-diagnosis, relative to 4-6 weeks post-diagnosis, in a group of children with provoked VTE who were enrolled in a single-institution prospective inception cohort study.
Objectives:The objectives of this work were:(1) toevaluate the prevalence of relative rebound hypercoagulability at 3 months status-post acute VTE in patients <21 years old; and (2) to investigate an association of relative rebound hypercoagulability with recurrent VTE.
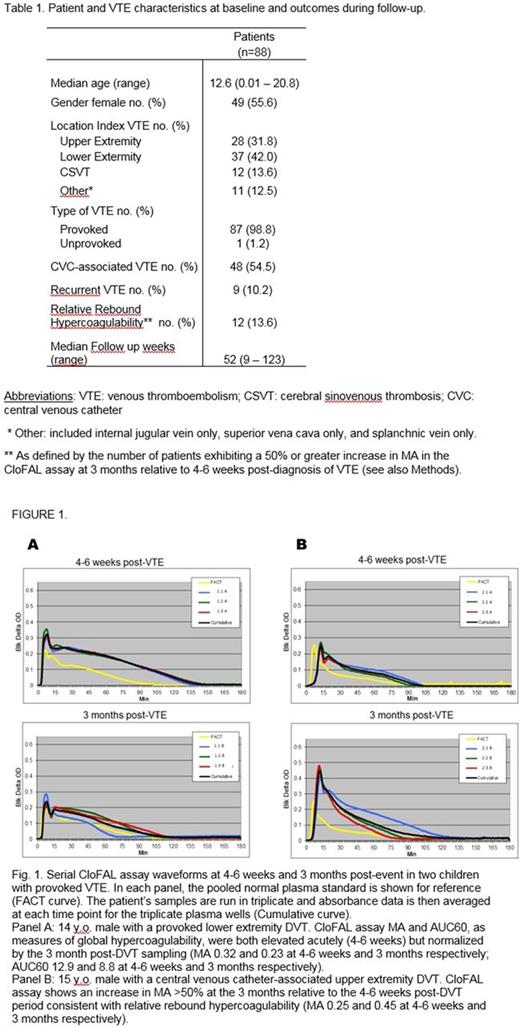
Methods:We combined clinical data and banked plasma biospecimens from an ongoing NHLBI-sponsored multinational multicenter trial of VTE treatment in patients <21 years old (the Kids-DOTT trial) with those from a single-institutional prospective cohort study of pediatric VTE at Johns Hopkins All Children's Hospital (St. Petersburg, FL, USA). The clot formation and lysis (CloFAL) spectrophotometric assay was performed as previously described, on banked plasma samples from 4-6 weeks and 3 months post-diagnosis of acute VTE, with heparinase pre-treatment of samples prior to assay. Those few patients (n=3) who received agents other than low molecular weight heparin at these time points were excluded. Assay measurements included maximal amplitude of the CloFAL waveform (MA), time to maximal amplitude (T1), and area under the curve at 60 minutes, indexed to that of the pooled normal plasma standard (AUC60). For each patient, the change in each CloFAL parameter between the 3 months and the 4-6 week post-VTE time points was then calculated. We used descriptive statistics to summarize data on patient characteristics VTE presentation, treatment, and outcomes. To explore prognostic cutoffs for percent change in each parameter receiver operating characteristics curves were generated, and inter-assay coefficients of variation of the assay were also considered. Thresholds of 50% increase in MA or AUC60, or 50% decrease in T1, were ultimately selected as candidate phenotypes of relative rebound hypercoagulability. An association between relative rebound hypercoagulability and VTE recurrence was determined via univariate logistic regression, using odds ratios (OR) and 95% confidence intervals (CI), with two-sided alpha=0.05. The blind was maintained in the Kids-DOTT trial throughout data transfer and analysis.
Results: The final study population consisted of 88 patients, with a median age of 12.6 years (range, 0.01-20.8 years). VTE was classified as provoked in 98% of cases. Patient and VTE characteristics at baseline and outcomes during follow-up are shown in Table 1. Median follow up time was 52 weeks (range 9-123 weeks). Relative rebound hypercoagulability was found in 13% of the study population at 3 months post-diagnosis. Recurrent VTE developed in 10% of patients. Logistic regression analysis revealed that change in CloFAL AUC60 and T1 were not prognostic of VTE recurrence. However, relative rebound hypercoagulability as measured by change in MA was associated with a statistically significant, 4-fold increase in the risk of recurrent VTE (OR=4.47, 95%CI=1.05-19.0; P=0.04).
Conclusions:Relative rebound hypercoagulability, as measured by change in MA in the CloFAL assay, developed in 13% of a multicenter study population of patients <21 years of age with predominantly-provoked VTE, and was associated with a 4-fold increased risk of recurrent VTE. Future work should seek to investigate mechanisms of relative rebound hypercoagulability following a conventional course of anticoagulation in young VTE patients, and to substantiate findings of its prognostic significance for VTE recurrence, via a future multicenter validation cohort.
Acknowledgment:The Kids-DOTT trial is supported via dual U01 awards from NIH NHLBI (Clinical Coordinating Center U01, Goldenberg, PI; Data Coordinating Center U01, Hiatt, PI).
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal